A lot has happened this week! Project 1 successfully reached its goal and ended. Because of this, I’ve been assigned a 3rd project! In this project there have been issues with a device used in colonoscopies recently- a wire loop with a mesh net used to collect samples for biopsy has a tendency to delaminate or break. Not good!
This type of background information for Project 3 is called qualitative information- typically found by identifying problems through a market analysis. After market analysis, R&D engineers had to take the qualitative information and turn it into quantitative information. In what position does the device break? How much force can the device withstand before it breaks? Is one side of the loop stronger than the other side? R&D engineers are basically science detectives, and are trying to identify the areas of concern within the medical device. Once questions like I mentioned above are given the proper numerical specifications, the process engineers step in to make the solution happen.
This is where my project begins: I have the test protocol, and I need to develop a validation method to verify the way the test is run wont affect the data. It’s basically testing the test that checks to see if something passes or fails. This is important because we want to make sure our test is repeatable and reproducible with little variation in our results.
I began to write the test-checker, aka the VTMV (Variable Test Method Validation) document, but I soon realized I lacked some foundation and structural information behind these complex tests. Thankfully, one of my managers Felipe helped me out and gave me some great resources from Six Sigma.
I spent a day reading all I possibly could, and have a much better understanding of the “Hows and Whys” behind a variable test method validation system. Variation in performing the test is undesirable, and Six Sigma gave great insight on identifying and eliminating variation. For example, variation can come from operator error(for example, a test favors those who are right handed). This type of variation is a more straightforward fix in the design of the test. Other types of variation are more difficult to pinpoint, and that is why statistical software like Minitab is awesome.
On Friday I was able to do a dry run of the test method- the numbers aren’t official, but they give me a good idea of what I’m working with. Next week I anticipate a finished protocol so I can later submit it to a board for approval. After that, my test method validation protocol will be used to test the colonoscopy devices here at Coyol! See you next week- Pura Vida!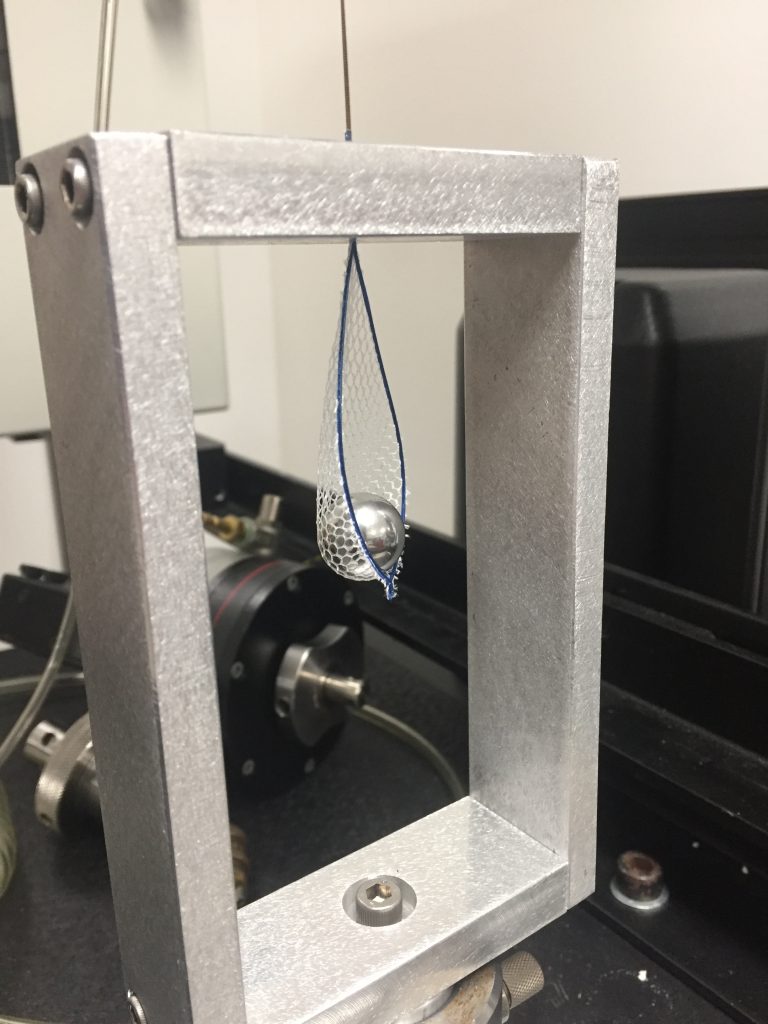

Pictured above is the setup for the test on a machine called an Instron. It measures the maximum tension used to break the mesh holding the metal ball (metaphorical biopsy sample) when pulled through a tiny hole symbolizing a catheter.